NanaBis - Drug Programme
Dr Sean Hall
The PAIN epidemic is real. We need to think differently. We need to innovate. We need to provide effective solutions.
For 65 million US adults, chronic pain is an issue requiring medical attention.
Medlab is advancing the development of NanaBis™, a multi-jurisdictional, patent-protected, non-opioid analgesic drug candidate that treats cancer and non-cancer pain designed to compete directly against the US$42 billion global opioid market. NanaBis™ is , initially designated for the treatment cancer bone pain but is expected to have broader applications across oncology and non-cancer pain indications.
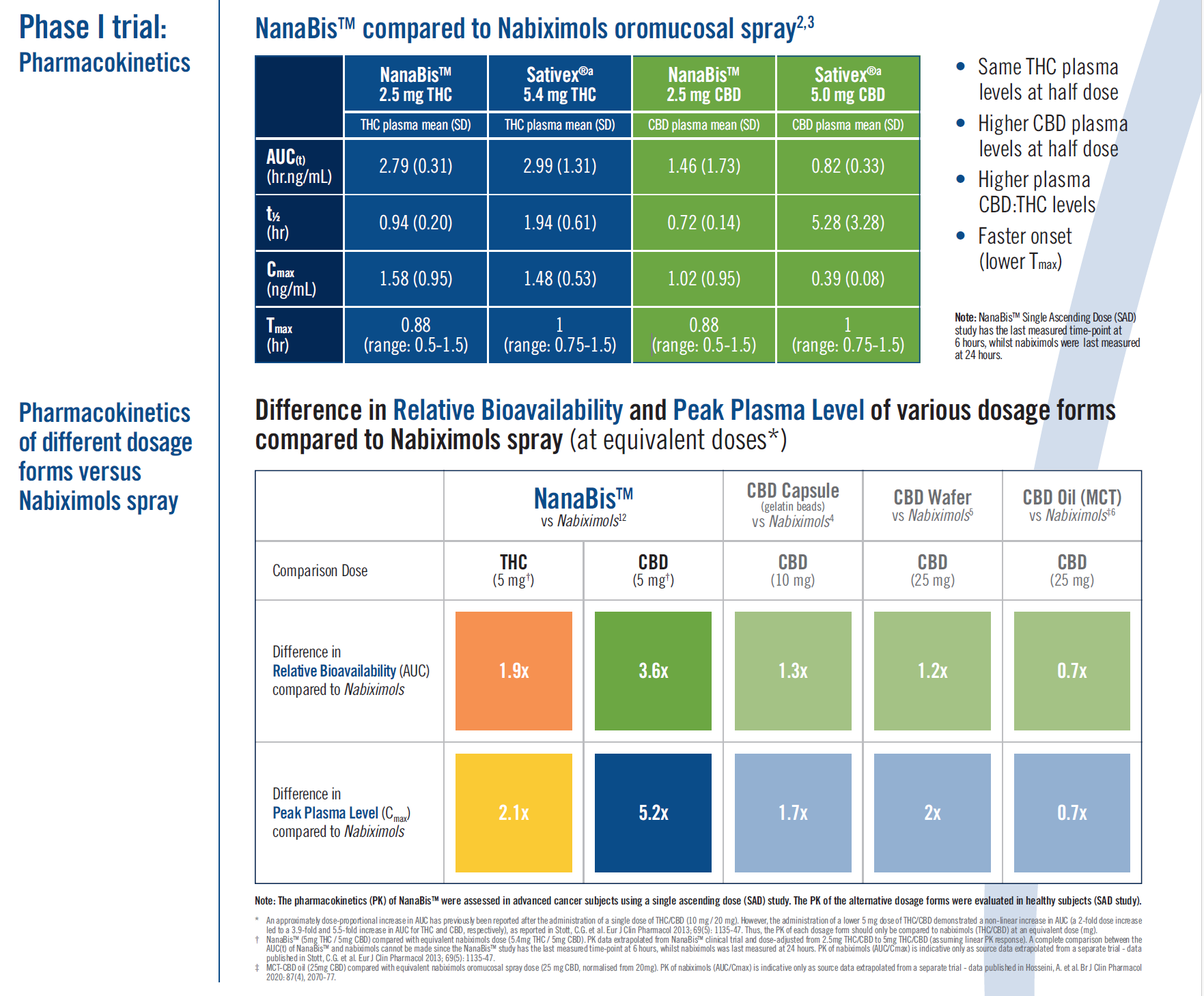
NanaBis™ consists of a synthetic mixture of CBD and delta-9-THC in a 1:1 ratio of 9.62 mg/mL CBD to 9.62 mg/mL THC. The formulation is a synthetic, standardized 1:1 blend of CBD and dronabinol, the delta 9 isomer of THC, in a patented submicron particle delivery platform (NanoCelle) optimized for buccal delivery.
Unlike opioids, NanaBis™ is a NanoCelle-delivered, synthetic, equimolar formulation comprising CBD and THC (Dronabinol), with its origins from standardized phyto-material, specifically targeting pain. Historically, NanaBis™ has undergone extensive clinical work, resulting in multiple publications, including published pharmacokinetic comparisons to Nabiximols at equivalent doses.
Published work shows NanaBis used in patients with advanced cancer and intractable pain, provided superior pain relief ~40% reduction (p<0.01) in patients with bone metastases, improvements in quality of life (QoL) and significant reductions in morphine milli equivalent.
Its opioid-sparing capability is designed to provide clinicians with an alternative approach to managing severe pain without well-documented risks of traditional opioids. In addition, its safety profile, particularly the absence of CARPA (a concern with some nanoparticle systems), may support potential long-term use. Based on these attributes, we believe NanaBis™ may represent a viable candidate for regulatory approval and, if approved, may have applicability in broader pain management settings. We believe these factors could make NanaBis™ an attractive therapeutic option for physicians and patients subject to the outcome of ongoing and future clinical studies and regulatory processes. If ultimately approved, NanaBis™ could compete in the multi-billion dollar global pain management market.
Ethics approved, first in man, Phase 1/2 outcomes were impressive amnd subsequently published:
With extended PK modelling by the University of South Australia, published:
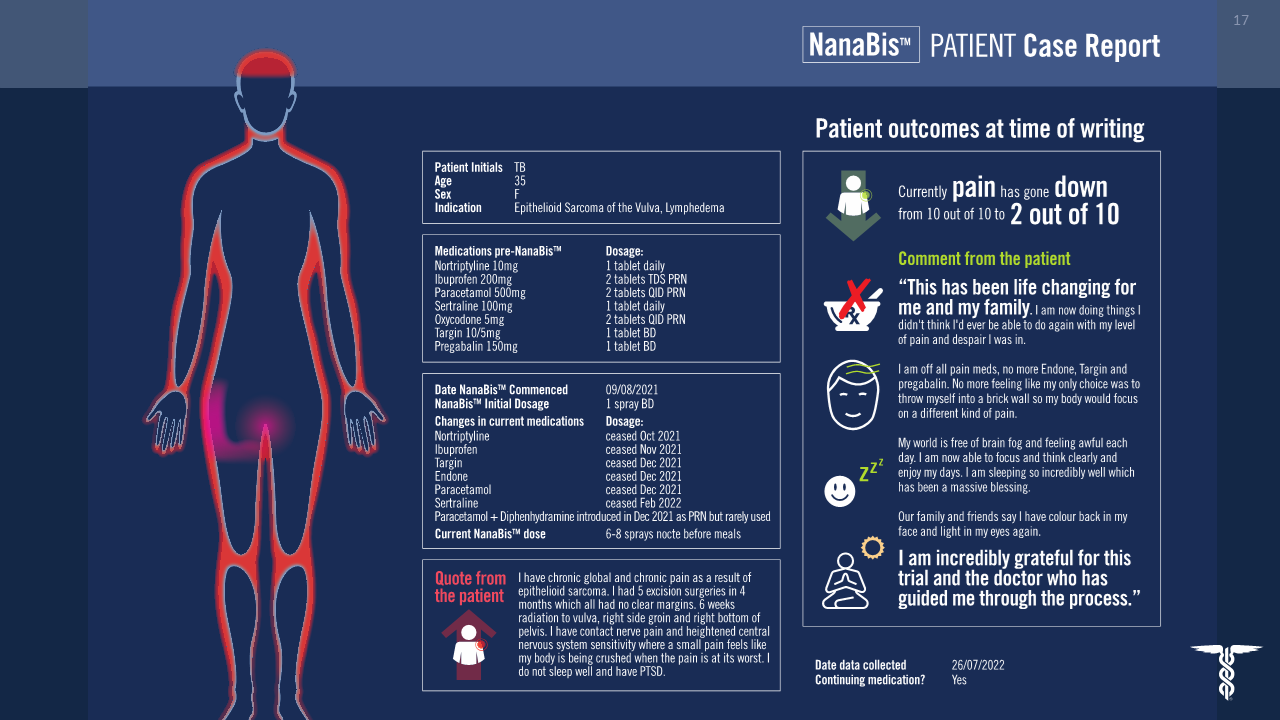
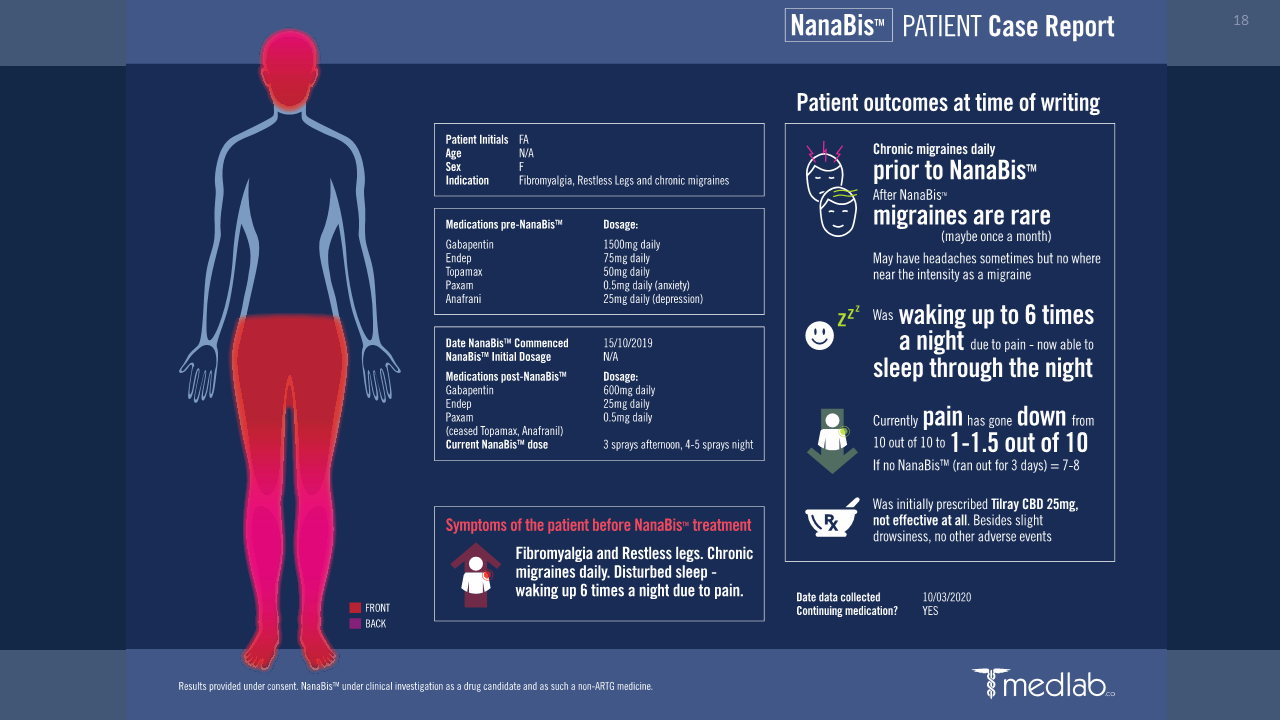
Ethics approved, RWE data across ~1200 subjects shows alignment to Phase 1/2 findings.
PROGRESSION STATUS
Principle Agency: FDA
Proposed Pathway: 505(b)(2)
Initial Indication: Cancer Induced Bone Pain
Claim Expansion: Nociplastic Pain
Next: awaiting guidance from FDA
Next Trial: n/a
EA-IND : Applications closed at this time
Physician Led IND: Applications closed at this time